Metal ions present in plasma are expected to: – Metal ions present in plasma are expected to play a multifaceted role in biological processes, enzyme activity, oxidative stress pathways, and clinical applications. Their interactions with proteins and their involvement in various physiological functions make them essential for maintaining homeostasis and overall well-being.
The presence of specific metal ions, such as sodium, potassium, calcium, and magnesium, in plasma is crucial for maintaining electrolyte balance, regulating nerve and muscle function, and supporting enzyme reactions. These ions are involved in a wide range of cellular processes, including energy production, cell signaling, and muscle contraction.
Metal Ions in Plasma: Their Role in Biological Processes
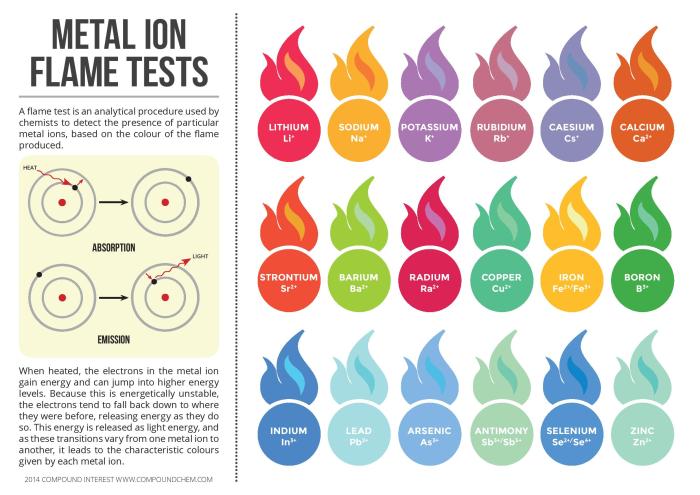
Metal ions are essential components of plasma, the liquid portion of blood, and play crucial roles in various biological processes. They contribute to maintaining the osmotic balance, pH levels, and overall homeostasis of the body.
Specific metal ions found in plasma include sodium, potassium, calcium, magnesium, iron, and zinc. Each of these ions has distinct functions:
- Sodium and potassium ions regulate fluid balance and electrical potential across cell membranes.
- Calcium ions are essential for bone formation, muscle contraction, and nerve function.
- Magnesium ions participate in enzyme reactions, muscle function, and energy production.
- Iron ions are vital for oxygen transport and red blood cell production.
- Zinc ions are involved in immune function, wound healing, and cell growth.
Imbalances in metal ion concentrations can disrupt biological systems, leading to various health conditions. For example, low sodium levels (hyponatremia) can cause fatigue, seizures, and coma. High calcium levels (hypercalcemia) can result in kidney stones, bone loss, and confusion.
Metal Ions and Protein Binding
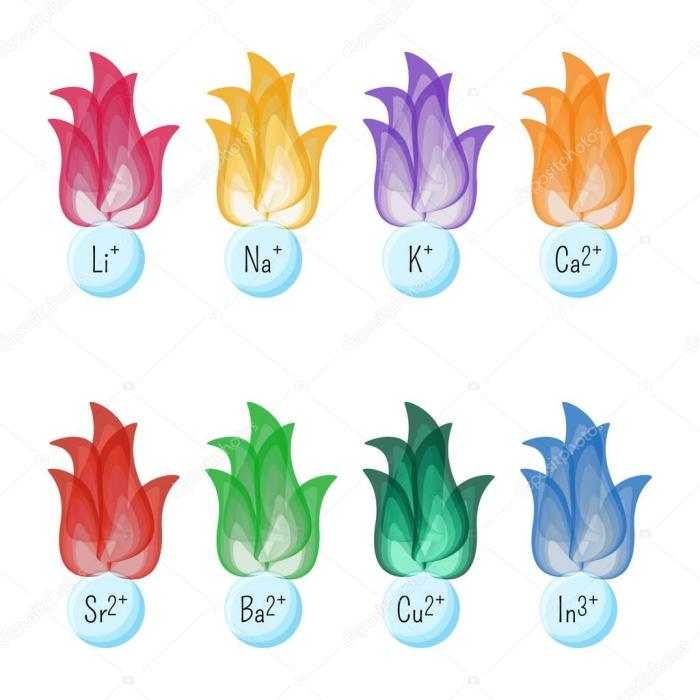
Metal ions interact with proteins in plasma, forming complexes that regulate their stability, function, and localization. This binding can occur through various mechanisms, including coordination bonds, electrostatic interactions, and hydrophobic interactions.
Proteins that bind to metal ions include:
- Albumin: Binds to various metal ions, including copper, zinc, and iron, and transports them throughout the body.
- Transferrin: Binds to iron ions and transports them to cells for hemoglobin synthesis.
- Ceruloplasmin: Binds to copper ions and plays a role in iron metabolism.
Metal ion binding to proteins can modulate their biological activity. For instance, zinc ions stabilize the structure of insulin, promoting its binding to receptors and enhancing its glucose-lowering effects.
Metal Ions and Enzyme Activity
Metal ions act as cofactors in enzyme reactions, facilitating the catalytic activity of enzymes. They participate in various enzyme mechanisms, including:
- Coordination of substrates to the enzyme’s active site.
- Stabilization of the transition state during the reaction.
- Redox reactions involving electron transfer.
Examples of enzymes that require metal ions for their activity include:
- Cytochrome oxidase (copper ions): Involved in cellular respiration.
- Alcohol dehydrogenase (zinc ions): Metabolizes alcohol.
- Superoxide dismutase (copper and zinc ions): Protects cells from oxidative damage.
Metal ion deficiency or excess can impair enzyme function, leading to metabolic disorders, neurological problems, and other health issues.
Metal Ions and Oxidative Stress
Metal ions can participate in oxidative stress pathways, generating reactive oxygen species (ROS) through Fenton and Haber-Weiss reactions. ROS can damage cellular components, including lipids, proteins, and DNA.
Iron ions are particularly prone to generating ROS, as they can undergo redox cycling between ferrous (Fe 2+) and ferric (Fe 3+) states. This cycling can lead to the production of hydroxyl radicals, highly reactive species that can cause oxidative damage.
Oxidative stress induced by metal ions can contribute to various diseases, such as atherosclerosis, neurodegenerative disorders, and cancer.
Metal Ions in Clinical Applications
Metal ions have numerous clinical applications, both diagnostic and therapeutic:
- Diagnostic tests:Measurement of metal ion concentrations in blood, urine, or other bodily fluids can aid in diagnosing various conditions, such as electrolyte imbalances, anemia, and heavy metal poisoning.
- Therapeutic interventions:Metal ions are used in various treatments, including:
- Iron supplements for anemia.
- Calcium supplements for osteoporosis.
- Antioxidant therapies with metal ions (e.g., selenium, zinc) to combat oxidative stress.
However, it’s crucial to note that metal ion-based treatments should be administered with caution, as excessive or inappropriate use can lead to adverse effects.
General Inquiries: Metal Ions Present In Plasma Are Expected To:
What are the common metal ions found in plasma?
Sodium, potassium, calcium, and magnesium are among the most common metal ions found in plasma.
How do metal ions interact with proteins?
Metal ions can bind to proteins through various mechanisms, including electrostatic interactions, coordination bonds, and hydrophobic interactions.
What is the role of metal ions in enzyme activity?
Metal ions can act as cofactors in enzyme reactions, facilitating substrate binding, catalysis, and enzyme stability.