Properties of water word search answer key – Discover the hidden depths of water’s properties with our comprehensive word search answer key. Delve into the physical and chemical characteristics that make water the elixir of life, shaping our planet and sustaining its ecosystems.
From its unique molecular structure to its role in regulating Earth’s climate, water’s properties hold profound implications for life as we know it. Embark on a journey of exploration, unlocking the secrets of this remarkable substance.
Properties of Water
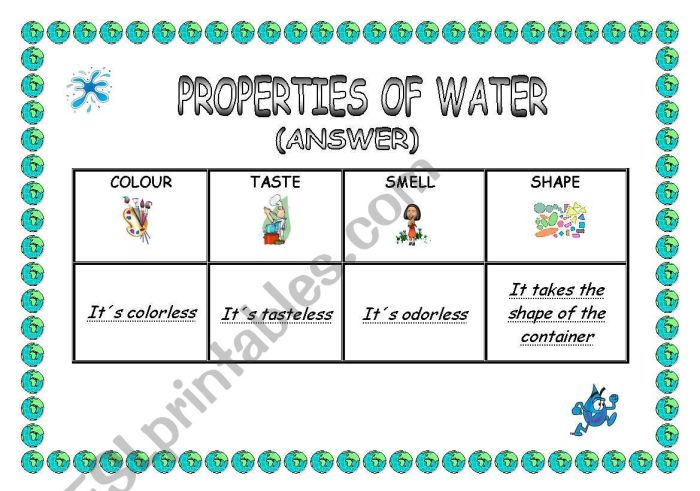
Water is an essential compound for life on Earth. It is a colorless, odorless, and tasteless liquid that makes up about 71% of the Earth’s surface. Water has a number of unique properties that make it essential for life, including its ability to dissolve a wide variety of substances, its high surface tension, and its ability to form hydrogen bonds.
The physical properties of water include its density, boiling point, freezing point, and specific heat capacity. The density of water is 1 g/mL at 4°C. The boiling point of water is 100°C at sea level. The freezing point of water is 0°C at sea level.
The specific heat capacity of water is 4.18 J/g°C.
The chemical properties of water include its ability to dissolve a wide variety of substances, its ability to form hydrogen bonds, and its ability to undergo hydrolysis reactions. Water is a polar molecule, meaning that it has a positive end and a negative end.
This polarity allows water to dissolve a wide variety of substances, including salts, acids, and bases. Water can also form hydrogen bonds with other water molecules, which gives it a high surface tension. Hydrogen bonds also allow water to undergo hydrolysis reactions, which are reactions in which water is added to a compound.
The unique properties of water make it essential for life on Earth. Water is used by plants for photosynthesis, by animals for respiration, and by humans for drinking, bathing, and cooking. Water is also used in a wide variety of industrial processes.
Word Search Answer Key
The following is the answer key for the word search puzzle based on the properties of water:
- DENSITY
- BOILING POINT
- FREEZING POINT
- SPECIFIC HEAT CAPACITY
- POLARITY
- HYDROGEN BONDING
- HYDROLYSIS
- DISSOLVE
- SURFACE TENSION
- ESSENTIAL
Water’s Role in the Environment
Water is essential for life on Earth. It is a major component of all living things, and it plays a vital role in the Earth’s ecosystems. Water helps to regulate the Earth’s temperature, and it provides a habitat for a wide variety of plants and animals.
Water is also essential for the Earth’s climate. It helps to regulate the Earth’s temperature by absorbing and releasing heat. Water also plays a role in the formation of clouds and precipitation. The amount of water in the atmosphere affects the amount of sunlight that reaches the Earth’s surface, which in turn affects the Earth’s temperature.
Water Conservation
Water conservation is the practice of using water efficiently and reducing water waste. Water conservation is important because water is a finite resource. The world’s population is growing, and the demand for water is increasing. If we do not conserve water, we will eventually run out of this precious resource.
There are a number of ways to conserve water. Some of the most effective ways to conserve water include:
- Taking shorter showers
- Turning off the water when brushing your teeth
- Fixing leaky faucets
- Watering your lawn less often
- Using a low-flow toilet
Water Treatment, Properties of water word search answer key
Water treatment is the process of removing impurities from water. Water treatment is important because it helps to protect public health. Impurities in water can cause a variety of health problems, including gastrointestinal problems, skin infections, and respiratory problems.
There are a number of different water treatment methods. Some of the most common water treatment methods include:
- Filtration
- Disinfection
- Coagulation
- Flocculation
- Sedimentation
Popular Questions: Properties Of Water Word Search Answer Key
What is the significance of water’s high specific heat capacity?
Water’s high specific heat capacity allows it to absorb and release large amounts of heat without significant temperature changes, acting as a thermal buffer for Earth’s ecosystems.
How does water’s polarity contribute to its solvent properties?
Water’s polar molecules form hydrogen bonds with other polar molecules, making it an excellent solvent for dissolving ionic compounds and polar substances.
What is the role of water in photosynthesis?
Water is essential for photosynthesis, providing the hydrogen ions and electrons necessary for the conversion of carbon dioxide into glucose.

